Suppliers
Contact Us
Voortstraat 49, 1910 Kampenhout BELGIUM Tel 0032 16 58 90 45 Fax 0032 16 50 90 45 This email address is being protected from spambots. You need JavaScript enabled to view it.">This email address is being protected from spambots. You need JavaScript enabled to view it. |
![]() GENTAUR BULGARIA
GENTAUR BULGARIA
53 Iskar Str. 1191 Kokalyane, Sofia
Tel 0035924682280
Fax 0035929830072
This email address is being protected from spambots. You need JavaScript enabled to view it." style="">This email address is being protected from spambots. You need JavaScript enabled to view it.
![]() GENTAUR France SARL
GENTAUR France SARL
9, rue Lagrange, 75005 Paris
Tel 01 43 25 01 50
Fax 01 43 25 01 60
This email address is being protected from spambots. You need JavaScript enabled to view it." style="">This email address is being protected from spambots. You need JavaScript enabled to view it.
This email address is being protected from spambots. You need JavaScript enabled to view it." style="">This email address is being protected from spambots. You need JavaScript enabled to view it.
![]() GmbH Marienbongard 20
GmbH Marienbongard 20
52062 Aachen Deutschland
Tel (+49) 0241 56 00 99 68
Fax (+49) 0241 56 00 47 88 This email address is being protected from spambots. You need JavaScript enabled to view it." style="font-family: Arial, Tahoma, Verdana, Helvetica; line-height: 15.59375px; ">
This email address is being protected from spambots. You need JavaScript enabled to view it." style="">This email address is being protected from spambots. You need JavaScript enabled to view it.
This email address is being protected from spambots. You need JavaScript enabled to view it." style="font-size: 12px; line-height: 1.3em;">
This email address is being protected from spambots. You need JavaScript enabled to view it." style="">This email address is being protected from spambots. You need JavaScript enabled to view it.
This email address is being protected from spambots. You need JavaScript enabled to view it.
![]() GENTAUR Ltd.
GENTAUR Ltd.
Howard Frank Turnberry House
1404-1410 High Road
Whetstone London N20 9BH
Tel 020 3393 8531
Fax 020 8445 9411
This email address is being protected from spambots. You need JavaScript enabled to view it." style="">This email address is being protected from spambots. You need JavaScript enabled to view it.
![]() GENTAUR Poland Sp. z o.o.
GENTAUR Poland Sp. z o.o.
ul. Grunwaldzka 88/A m.2
81-771 Sopot, Poland
Tel 058 710 33 44
Fax 058 710 33 48
This email address is being protected from spambots. You need JavaScript enabled to view it." style="">This email address is being protected from spambots. You need JavaScript enabled to view it.
![]() GENTAUR Nederland BV
GENTAUR Nederland BV
Kuiper 1
5521 DG Eersel Nederland
Tel 0208-080893
Fax 0497-517897
This email address is being protected from spambots. You need JavaScript enabled to view it." style="">This email address is being protected from spambots. You need JavaScript enabled to view it.
![]() GENTAUR SRL IVA IT03841300167
GENTAUR SRL IVA IT03841300167
Piazza Giacomo Matteotti, 6, 24122 Bergamo
Tel 02 36 00 65 93
Fax 02 36 00 65 94
This email address is being protected from spambots. You need JavaScript enabled to view it.">This email address is being protected from spambots. You need JavaScript enabled to view it.
![]() GENTAUR Spain
GENTAUR Spain
Tel 0911876558
This email address is being protected from spambots. You need JavaScript enabled to view it." style="">This email address is being protected from spambots. You need JavaScript enabled to view it.
![]() Genprice Inc, Logistics
Genprice Inc, Logistics
547, Yurok Circle
San Jose, CA 95123
Phone/Fax:
(408) 780-0908
This email address is being protected from spambots. You need JavaScript enabled to view it.
GENPRICE Inc. invoicing/ accounting:
6017 Snell Ave, Suite 357
San Jose, CA. 96123
![]() Serbia,
Serbia, ![]() Macedonia,
Macedonia,
![]() Montenegro,
Montenegro, ![]() Croatia:
Croatia:
Tel 0035929830070
Fax 0035929830072
This email address is being protected from spambots. You need JavaScript enabled to view it.">This email address is being protected from spambots. You need JavaScript enabled to view it.
![]() GENTAUR Romania
GENTAUR Romania
Tel 0035929830070
Fax 0035929830072
This email address is being protected from spambots. You need JavaScript enabled to view it.">This email address is being protected from spambots. You need JavaScript enabled to view it.
![]() GENTAUR Greece
GENTAUR Greece
Tel 00302111768494
Fax 0032 16 50 90 45
This email address is being protected from spambots. You need JavaScript enabled to view it.">This email address is being protected from spambots. You need JavaScript enabled to view it.
Other countries
Luxembourg +35220880274
Schweiz Züri +41435006251
Danmark +4569918806
Österreich +43720880899
Ceská republika Praha +420246019719
Ireland Dublin +35316526556
Norge Oslo +4721031366
Finland Helsset +358942419041
Sverige Stockholm +46852503438
Magyarország Budapest +3619980547
Avian Influenza Virus Detection Using Smell
 New research from the Monell Chemical Senses Center and the U.S. Department of Agriculture (USDA) reveals how diseases can modify animal odors in subtle ways. In a recent study published in the public access journal PLOS ONE, scientists examined how infection with avian influenza (AIV) alters fecal odors in mallards.
New research from the Monell Chemical Senses Center and the U.S. Department of Agriculture (USDA) reveals how diseases can modify animal odors in subtle ways. In a recent study published in the public access journal PLOS ONE, scientists examined how infection with avian influenza (AIV) alters fecal odors in mallards.
Using both behavioral and chemical methods, the findings reveal that AIV can be detected based on odor changes in infected birds.
"The fact that a distinctive fecal odor is emitted from infected ducks suggests that avian influenza infection in mallards may be 'advertised' to other members of the population," notes Bruce Kimball, PhD, a research chemist with the USDA National Wildlife Research Center (NWRC) stationed at the Monell Center. "Whether this chemical communication benefits non-infected birds by warning them to stay away from sick ducks or if it benefits the pathogen by increasing the attractiveness of the infected individual to other birds, is unknown."
In the study, laboratory mice were trained to discriminate between feces from AIV-infected and non-infected ducks, indicating a change in odor. Chemical analysis then identified the chemical compounds associated with the odor changes as acetoin and 1-octen-3-ol.
The same compounds also have been identified as potential biomarkers for diagnosing gastrointestinal diseases in humans. Kimball and colleagues hypothesize that metabolites resulting from viral infection interact in concert with bacteria in the gastro-intestinal system of ducks to produce "odor signatures" indicating presence of the AI virus.
"Avian influenzas are typically asymptomatic in ducks and waterfowl. Infection in these species can only be diagnosed by directly detecting the virus, requiring capture of birds and collection of swab samples. Our results suggest that rapid and simple detection of influenzas in waterfowl populations may be possible through exploiting this odor change phenomenon," said Monell behavioral biologist Gary Beauchamp, PhD, also an author on the paper.
Future work will assess whether odor changes can be used for surveillance of AIV in waterfowl. In particular, researchers are interested in whether the odor change is specific to the AIV pathogen or if it is merely a general response to a variety of pathogens normally found in birds. Other studies will explore communicative functions of the AIV odor to gain greater understanding of how odors can shape social behavior in wildlife populations.
Also contributing to the research, which was funded by the National Wildlife Research Center, were Kunio Yamazaki and Maryanne Opiekun of Monell and Richard Bowen and Jack Muth from Colorado State University. Dr. Yamazaki, who actively contributed to the design and realization of this work, died in April 2103.
Researchers create accurate computer model of RNA tetraloop
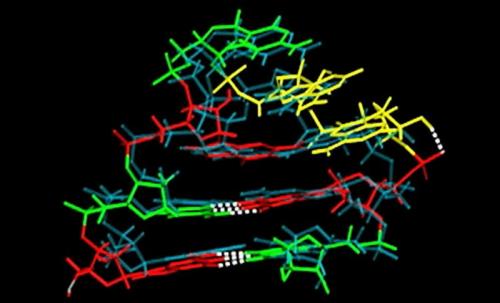
A computational model developed by researchers at Rensselaer Polytechnic Institute is the first to accurately simulate the complex twists of a short sequence of RNA as it folds into a critical hairpin structure known as a "tetraloop." The research, published today in Proceedings of the National Academy of Sciences, is a glimpse into RNA, found in all life on Earth, and could advance a variety of research areas, including the search for new antibiotics and cures for protein-related diseases.
Existing computational models, based on DNA rather than RNA, do not achieve the atomic level accuracy of the new model, said Angel Garcia, head of the Department of Physics, Applied Physics, and Astronomy within the School of Science at Rensselaer, and senior constellation chaired professor in the Biocomputation and Bioinformatics Constellation, who co-wrote the paper with Alan Chen, a post-doctoral fellow at Rensselaer. The new model Garcia and Chen created can simulate the folding of three known versions of a tetraloop, accurate to within one ten-billionth of a meter.
RNA is involved in many biological functions, such as building proteins, coding and decoding genes, and cellular regulation. RNA molecules are composed of strings of four different "bases" —cytosine, guanine, adenine, and uracil—mounted on a sugar-phosphate backbone. Once the sequence is assembled, the individual bases interact with their neighbors, twisting and swinging on the hinged chemical bonds that connect them to the backbone. When the process is complete, the RNA has folded into its "tertiary" structure, which influences its function. Although researchers can easily alter the sequence of molecules, without accurate computer modeling there they cannot easily see the tertiary structure of their creation.
"Right now, it takes people from molecular biologists, to virologists, to cell biologists, thousands of dollars and years of study to see the structure of an RNA they have made, altered, or are studying," said Chen. "There are a lot of researchers working on the RNA in viruses and how it attacks the cell, and, while they're easily able to alter the sequence, they're essentially working without ever seeing the effects of their changes in molecular detail. Because of this, there's a lot of trial and error, and our work aimed at helping that."
Garcia and Chen said that, unlike DNA, which typically twists two strands of bases into a classic double-helix, RNA is single-stranded and folds onto itself, forming many unusual structures. A tetraloop is a small section of single-stranded RNA that is looped into the shape of a hairpin, the curve of which is formed by four bases. Even the sequence of bases in a tetraloop is unusual, violating a standard arrangement described by groundbreaking DNA researchers James Watson and Francis Crick.
To create an effective computational model, Garcia and Chen had to match the unique "recipe" of twisting and swinging proscribed by the interactions between the bases.
"Imagine if you try to produce a recipe of Mario Batali," said Garcia, referring to a popular chef. "I tell you it has water, salt, fish, and pasta—go produce his recipe. The problem is, you don't know how much of each, and in what order."
Instead of a recipe of food ingredients, Garcia and Chen created a computational recipe for the interactions of the bases in the sequence of a tetraloop.
"The problem is one of balancing different forces. It's the actions between the bases as they stack on top of each other, the interactions of the bases with water, the rotation of the bases relative to a sugar. Those are things that change the balance," said Garcia.
Garcia said tetraloops are an important area of study because they appear in all organisms, particularly in ribosomes, which manufacture proteins for living cells. Statistically, there could be as many as 256 possible sequences of those four bases, but only three sequences actually appear in tetraloops. Once formed, they are highly stable, outlasting other structures when subjected to the destructive force of increasing heat.
"Tetraloops are sequences which are highly conserved throughout evolution; you find them everywhere, from bacteria to humans," said Garcia. "From one organism to another, many things can change, but when tetraloops change, they change from one sequence of four bases to one of the other three. They stack against each other and they are hyperstable. And there is a reason for them to be arranged the way they are."
Pain-Free Microneedle Influenza Vaccine Is Effective, Long-Lasting
 Microneedles is a medium for supply of influenza vaccine, which avoids the pain associated with conventional needles. They are only seven tenths of a millimeter, and the volume of vaccine - an essential factor in pain - is small.
Microneedles is a medium for supply of influenza vaccine, which avoids the pain associated with conventional needles. They are only seven tenths of a millimeter, and the volume of vaccine - an essential factor in pain - is small.
Instead of liquid, whole killed or attenuated viruses, using dry vaccines virus-like particles (VLPs) that simply coating the needles in the presence of the basic stabilizer, eliminating the need for cooling - the possible handover for use in developing countries.The lower dose required when using microneedles also reduces the potential for side effects, such as lung inflammation.
"This method can induce higher levels of IgG2a antibody and quick recall to elicit an immune response to infection lethal. Our previous studies showed that the microneedle vaccination enhances antibody-producing cells in the spleen and bone marrow induced compared to intramuscular vaccination" says Sang Moo Kang Georgia State University, researcher of the study.
Previous studies by this group has shown that an influenza VLP-coated microneedles indeed produce higher short-term protection than traditional intramuscular immunization. In this study, researchers tested how effective long-term protection of the vaccine. Mice that received the vaccine were 100 percent protected against a lethal challenge with influenza virus 14 months after vaccination.
Kang says that his goal was to develop a simple and painless method of administration of vaccines. He also says that the patient would probably use this system to be vaccinated.
The human body strengthens protection against influenza throughout life
 A natural reaction to the pandemic influenza virus - an ideal model, suitable for a universal flu vaccine. According to Live Science, researchers specifically examined the changes in the immune system caused by constant exposure to the virus of influenza. Analysis was applied to blood samples from 40 people 35-70 years of age.
A natural reaction to the pandemic influenza virus - an ideal model, suitable for a universal flu vaccine. According to Live Science, researchers specifically examined the changes in the immune system caused by constant exposure to the virus of influenza. Analysis was applied to blood samples from 40 people 35-70 years of age.
People are faced with two strains of the pandemic virus (H2N2 - in 1957 and H1N1 - in 1977), had elevated levels of immune proteins - a broad spectrum neutralizing antibodies. These antibodies attack the part of the virus, called a "trunk". It differs only slightly depending on the strain. But the "head" of the virus changes frequently. If you find a way to increase the concentration of these antibodies, you get a new vaccine against influenza.
However, such antibodies do not normally produced in large quantities when in contact with the seasonal influenza. The body realizes that it is now important to produce antibodies that attack the "head" of the virus.
And only if the virus is very different from the previous ones by the structure of the "head" (it comes with a pandemic strain), the body begins to increase the concentration of neutralizing a broad spectrum of antibodies that work against the "trunk". The aim - to create a vaccine conditions similar to those that are added when pandemic influenza.
The highest concentration of neutralizing antibodies in humans was facing more than one pandemic. If a person is in contact with H2N2, and H1N1, the figures are higher by 3.8 times compared to a person, only bolevshim H1N1.
The level of antibodies targeted at the "head" of the virus, eventually grew - despite the fact that the contact with the pandemic virus was only once. From this, scientists have concluded that immunity to this strain of flu remains active for a long time. And in fact, the body is constantly enhances protection against strains with which the person met.
Buy Influenza products form Gentaur:
INFLUENZA B VIRUS (Texas/6/11) Infectious Culture Fluid
 PRODUCT DESCRIPTION:
PRODUCT DESCRIPTION:
Influenza viruses are enveloped viruses with a diameter of 80-120 nm, and contain a singlestranded, segmented, negative-sense RNA within a nucleocapsid. Influenza virus is propagated in the MDCK cell line. Influenza Culture Fluid is sold in 1.0 mL aliquots, and is shipped on dry ice. Viral culture fluids consist of virus, cells, and media taken directly from the tissue culture flask. Each lot of viral culture fluid is assayed for its Tissue Culture Infective Dose (TCID50), and sold with titers >105 U/ml. Custom orders are available, including specific titers and package sizes.
INTENDED USE:
This product is intended for research, product development testing, or quality assurance testing. Viral culture fluids are sold as consumable testing materials, and are not for propagation or commercialization. Applications include:
- Nucleic Acid / Molecular Testing
- Limit of Detection (LOD) Studies
- Cross-reactivity Studies
- Other Viral-based Assays
TIOLOGIC STATUS/BIOHAZARD TESTING:
Influenza virus is a Biosafety Level 2 organism.
PRECAUTIONS:
USE UNIVERSAL PRECAUTIONS when handling this product! Viral Culture Fluid is live and infectious!! This material should be handled as if capable of transmitting infectious agents.
RECOMMENDED STORAGE:
Viral culture fluid is stable for at least one year when stored at -65ºC or below. To avoid repeat freeze-thaws, which could negatively impact product performance, culture fluid should be stored in aliquots upon receipt.
DO NOT USE IN HUMANS!
These products are NOT intended for use in the manufacture or processing of injectable products subject to licensure under section 351 of the Public Health Service Act, or for any other product intended for administration to humans.
INFLUENZA B VIRUS (Wisconsin/1/10) Infectious Culture Fluid
 PRODUCT DESCRIPTION:
PRODUCT DESCRIPTION:
Influenza viruses are enveloped viruses with a diameter of 80-120 nm, and contain a singlestranded, segmented, negative-sense RNA within a nucleocapsid. Influenza virus is propagated in the MDCK cell line. Influenza Culture Fluid is sold in 1.0 mL aliquots, and is shipped on dry ice. Viral culture fluids consist of virus, cells, and media taken directly from the tissue culture flask. Each lot of viral culture fluid is assayed for its Tissue Culture Infective Dose (TCID50), and sold with titers >105 U/ml. Custom orders are available, including specific titers and package sizes.
INTENDED USE:
This product is intended for research, product development testing, or quality assurance testing. Viral culture fluids are sold as consumable testing materials, and are not for propagation or commercialization. Applications include:
- Nucleic Acid / Molecular Testing
- Limit of Detection (LOD) Studies
- Cross-reactivity Studies
- Other Viral-based Assays
TIOLOGIC STATUS/BIOHAZARD TESTING:
Influenza virus is a Biosafety Level 2 organism.
PRECAUTIONS:
USE UNIVERSAL PRECAUTIONS when handling this product! Viral Culture Fluid is live and infectious!! This material should be handled as if capable of transmitting infectious agents.
RECOMMENDED STORAGE:
Viral culture fluid is stable for at least one year when stored at -65ºC or below. To avoid repeat freeze-thaws, which could negatively impact product performance, culture fluid should be stored in aliquots upon receipt.
DO NOT USE IN HUMANS!
These products are NOT intended for use in the manufacture or processing of injectable products subject to licensure under section 351 of the Public Health Service Act, or for any other product intended for administration to humans.
INFLUENZA A H3 VIRUS (Victoria/361/11) Infectious Culture Fluid
 PRODUCT DESCRIPTION:
PRODUCT DESCRIPTION:
Influenza viruses are enveloped viruses with a diameter of 80-120 nm, and contain a singlestranded, segmented, negative-sense RNA within a nucleocapsid. Influenza virus is propagated in the MDCK cell line. Influenza Culture Fluid is sold in 1.0 mL aliquots, and is shipped on dry ice. Viral culture fluids consist of virus, cells, and media taken directly from the tissue culture flask. Each lot of viral culture fluid is assayed for its Tissue Culture Infective Dose (TCID50), and sold with titers >105 U/ml. Custom orders are available, including specific titers and package sizes.
INTENDED USE:
This product is intended for research, product development testing, or quality assurance testing. Viral culture fluids are sold as consumable testing materials, and are not for propagation or commercialization. Applications include:
- Nucleic Acid / Molecular Testing
- Limit of Detection (LOD) Studies
- Cross-reactivity Studies
- Other Viral-based Assays
TIOLOGIC STATUS/BIOHAZARD TESTING:
Influenza virus is a Biosafety Level 2 organism.
PRECAUTIONS:
USE UNIVERSAL PRECAUTIONS when handling this product! Viral Culture Fluid is live and infectious!! This material should be handled as if capable of transmitting infectious agents.
RECOMMENDED STORAGE:
Viral culture fluid is stable for at least one year when stored at -65ºC or below. To avoid repeat freeze-thaws, which could negatively impact product performance, culture fluid should be stored in aliquots upon receipt.
DO NOT USE IN HUMANS!
These products are NOT intended for use in the manufacture or processing of injectable products subject to licensure under section 351 of the Public Health Service Act, or for any other product intended for administration to humans.
INFLUENZA B VIRUS (Massachusetts/2/12) Infectious Culture Fluid
 PRODUCT DESCRIPTION:
PRODUCT DESCRIPTION:
Influenza viruses are enveloped viruses with a diameter of 80-120 nm, and contain a singlestranded, segmented, negative-sense RNA within a nucleocapsid. Influenza virus is propagated in the MDCK cell line. Influenza Culture Fluid is sold in 1.0 mL aliquots, and is shipped on dry ice. Viral culture fluids consist of virus, cells, and media taken directly from the tissue culture flask. Each lot of viral culture fluid is assayed for its Tissue Culture Infective Dose (TCID50), and sold with titers >105 U/ml. Custom orders are available, including specific titers and package sizes.
INTENDED USE:
This product is intended for research, product development testing, or quality assurance testing. Viral culture fluids are sold as consumable testing materials, and are not for propagation or commercialization. Applications include:
- Nucleic Acid / Molecular Testing
- Limit of Detection (LOD) Studies
- Cross-reactivity Studies
- Other Viral-based Assays
TIOLOGIC STATUS/BIOHAZARD TESTING:
Influenza virus is a Biosafety Level 2 organism.
PRECAUTIONS:
USE UNIVERSAL PRECAUTIONS when handling this product! Viral Culture Fluid is live and infectious!! This material should be handled as if capable of transmitting infectious agents.
RECOMMENDED STORAGE:
Viral culture fluid is stable for at least one year when stored at -65ºC or below. To avoid repeat freeze-thaws, which could negatively impact product performance, culture fluid should be stored in aliquots upon receipt.
DO NOT USE IN HUMANS!
These products are NOT intended for use in the manufacture or processing of injectable products subject to licensure under section 351 of the Public Health Service Act, or for any other product intended for administration to humans.
INFLUENZA A H3 VIRUS (Texas/50/12) Infectious Culture Fluid
 PRODUCT DESCRIPTION:
PRODUCT DESCRIPTION:
Influenza viruses are enveloped viruses with a diameter of 80-120 nm, and contain a singlestranded, segmented, negative-sense RNA within a nucleocapsid. Influenza virus is propagated in the MDCK cell line. Influenza Culture Fluid is sold in 1.0 mL aliquots, and is shipped on dry ice. Viral culture fluids consist of virus, cells, and media taken directly from the tissue culture flask. Each lot of viral culture fluid is assayed for its Tissue Culture Infective Dose (TCID50), and sold with titers>105 U/ml. Custom orders are available, including specific titersand package sizes.
INTENDED USE:
This product is intended for research, product development testing, or quality assurance testing. Viral culture fluids are sold as consumable testing materials, and are not for propagation or commercialization. Applications include:
- Nucleic Acid / Molecular Testing
- Limit of Detection (LOD) Studies
- Cross-reactivity Studies
- Other Viral-based Assays
TIOLOGIC STATUS/BIOHAZARD TESTING:
Influenza virus is a Biosafety Level 2 organism.
PRECAUTIONS:
USE UNIVERSAL PRECAUTIONS when handling this product! Viral Culture Fluid is live and infectious!! This material should be handled as if capable of transmitting infectious agents.
RECOMMENDED STORAGE:
Viral culture fluid is stable for at least one year when stored at -65ºC or below. To avoid repeat freeze-thaws, which could negatively impact product performance, culture fluid should be stored in aliquots upon receipt.
DO NOT USE IN HUMANS!
These products are NOT intended for use in the manufacture or processing of injectable products subject to licensure under section 351 of the Public Health Service Act, or for any other product intended for administration to humans.
Influenza A H3 (Texas/50/12) Lysate
 PRODUCT DESCRIPTION:
PRODUCT DESCRIPTION:
Influenza viruses are enveloped viruses with a diameter of 80-120 nm, and contain a singlestranded, segmented, negative-sense RNA within a nucleocapsid. Influenza virus is propagated in the MDCK cell line. The virus is purified using sucrose density gradient
ultracentrifugation, disrupted in the presence of 0.5% Triton X-100 non-ionic detergent/0.6 M KCl, and heat inactivated. Influenza lysate is sold in vials containing 1.0 mg of protein, and is shipped on dry ice. Protein concentrations generally range from 0.5 to 3.0
mg/ml. Custom orders are available, including specific buffer formulations and package sizes.
INTENDED USE:
This product is intended for research, product development, quality assurance testing, or further manufacturing use. Viral lysates can be utilized as an antigen, as a source for the purification of viral proteins, or for the detection of viral antibodies.
Applications include:
- Immunodetection of antibodies to Influenza virus using solid-phase enzyme immunoassays (EIA)
- Western blot
- Dot blot
- Other protein-based assays
TIOLOGIC STATUS/BIOHAZARD TESTING:
Influenza virus is a Biosafety Level 2 organism. Viral inactivation is verified for every lot of lysate by the absence of viral growth in validated tissue culture based infectivity assays.
PRECAUTIONS:
USE UNIVERSAL PRECAUTIONS when handling this product! Influenza Lysate has been treated by a method validated to be effective for virus inactivation. However, no method can be guaranteed 100% effective. This material should be handled as if capable of transmitting infectious agents.
RECOMMENDED STORAGE:
The viral lysate is stable for at least one year when stored at -65ºC or below. To avoid repeat freeze-thaws, which could negatively impact product performance, viral lysateshould be stored in aliquots upon receipt.
DO NOT USE IN HUMANS!
These products are NOT intended for use in the manufacture or processing of injectable products subject to licensure under section 351 of the Public Health Service Act, or for any other product intended for administration to humans.


















